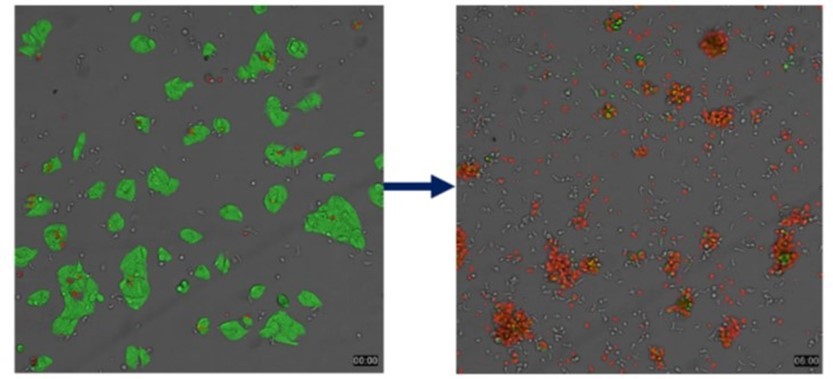
Autologous Magicell-NK:
R&D/Preclinical Phase I Clinical Trial Phase II Clinical Trial Phase III Clinical TrialMarket Approval
Title
A Dose-Escalating Phase I Study to Determine the Safety, and Maximum Tolerated Dose/ Maximum Feasible Dose of Autologous ex vivo Expanded and Activated NK cell, Magicell-NK, Infusion for Colon Cancer post Resection
Objectives
To evaluate the safety, dose-limiting toxicity (DLT) and Maximum Tolerated Dose (MTD) / Maximum Feasible Dose (MFD) of intravenous autologous NK cell therapy (Magicell-NK) in post-surgical patients with stage I or IIa colon cancer.
Enrollment Information
Planned Enrollment: 12 to 18 subjects
Estimated Enrollment Period: November 2021 to December 2026
Principal Investigator: Dr. Chun-Kai Liao
Site: Department of Colorectal Surgery, Chang Gung Memorial Hospital, Linkou
In 2021, a Phase I clinical trial (CT-NK-11) was initiated using a dose-escalation design to evaluate the safety and dose tolerance of autologous Magicell-NK in patients with resected colon cancer. The trial has successfully progressed to the enrollment stage of the third dose cohort (Cohort 3).
[ClinicalTrials.gov Identifier: NCT05394714]
Allogeneic Magicell-NK:
R&D/Preclinical Phase I Clinical Trial Phase II Clinical Trial Phase III Clinical TrialMarket Approval
Title
A Dose-Finding Phase I Followed by a Phase II Study to Evaluate the Safety and Efficacy of Allogeneic NK-cell Therapy (ex vivo Expanded and Activated NK Cell, Allogeneic Magicell-NK), as Adjuvant Therapy Combined with Chemotherapy in Patients with Pancreatic Ductal Adenocarcinoma (PDA) or Cholangiocarcinoma after Surgery
Objectives
Phase I Clinical Trial:
To evaluate the safety, dose-limiting toxicity (DLT) and Maximum Tolerated Dose (MTD) / Maximum Feasible Dose (MFD) of intravenous allogeneic NK cell therapy (Magicell-NK) combined with chemotherapy for post-surgical patients with stage II or III Pancreatic Ductal Adenocarcinoma (PDA) or Cholangiocarcinoma.
Phase II Clinical Trial:
To evaluate the antitumor efficacy of intravenous allogeneic NK cell therapy (Magicell-NK) combined with chemotherapy for post-surgical patients with stage II or III Pancreatic Ductal Adenocarcinoma (PDA) or Cholangiocarcinoma.
Enrollment Information
Planned Enrollment:Phase I: 6 to 12 participants
Phase II: 30 participants
Estimated Enrollment Period: September 2024 to December 2029
Principal Investigator: Dr. Yan-Sheng Shen
Sites:Phase I: National Cheng Kung University Hospital (NCKUH)
Phase II: two to three hospitals (to be determined)
In 2024, a Phase I/II clinical trial (CT-ANK-21) was initiated to evaluate the safety and efficacy of allogeneic Magicell-NK cell therapy combined with chemotherapy as an adjuvant treatment for patients with resected Pancreatic Ductal Adenocarcinoma (PDA) or cholangiocarcinoma. The study adopts a dose-escalation followed by efficacy expansion design and is currently ongoing.
[ClinicalTrials.gov Identifier: NCT06730009]