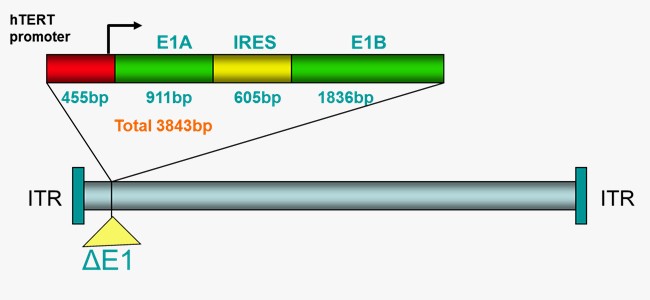
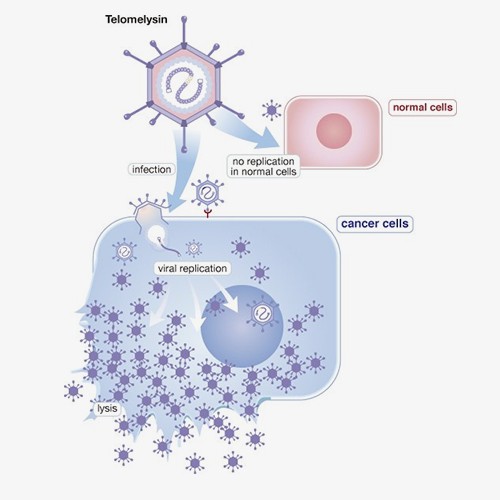
[Esophageal Cancer – Combined with Radiotherapy]
1. Safety and Efficacy: A researcher-initiated clinical study combining radiotherapy with OBP-301 for esophageal cancer was conducted at Okayama University in Japan. The study was completed in 2018. Among the 13 patients enrolled, 8 achieved complete response (CR), with CD8+ T cell infiltration observed in the tumor region.
Based on these encouraging results, Oncolys initiated a Phase I clinical trial in 2017. This study was completed in September 2019, with no dose-limiting toxicity (DLT) observed and the results confirmed a favorable safety profile.
▲ Presented at the European Society for Medical Oncology (ESMO) in September 2021.
2. A Phase II clinical trial of OBP-301 in combination with radiotherapy for esophageal cancer conducted in Japan began in March 2020 and completed the enrollment of the last subject in December 2022.
3. In the Phase II clinical trial of OBP-301 in combination with radiotherapy for esophageal cancer. The primary efficacy endpoint “Local Complete Response rate (L-CR)” exceeded the predefined threshold set in the clinical trial protocol, demonstrating the efficacy of OBP-301 in locally advanced esophageal cancer. According to the evaluation by the central endoscopic review committee, the L-CR rate was 41.7%, surpassing the predefined efficacy threshold of 30.2%. The secondary efficacy endpoint “Local Response Rate (L-RR), defined as cases where the primary tumor did not completely disappear but showed significant shrinkage” was 16.7%. Together, the combined local response rate (L-CR + L-RR) reached 58.3%. As of now, the one-year survival rate is 71.4%, which is higher than the 57.4% one-year survival rate for radiotherapy alone reported in the nationwide registry data from the Japan Esophageal Society. The main adverse events related to OBP-301 were mild to moderate in severity and transient in nature.
▲ Announced on October 16, 2023
4. Oncolys BioPharma and the Pharmaceuticals and Medical Devices Agency (PMDA) of Japan initiated the “Sakigake Comprehensive Assessment Consultation”*, with the goal of submitting the approval application for OBP-301 by the end of December of the same year.
▲ Announced on March 18, 2025
5. Oncolys BioPharma and the Pharmaceuticals and Medical Devices Agency (PMDA) of Japan initiated the “Sakigake Comprehensive Assessment Consultation”*. In May, notable progress was made on the “Non-clinical” and“Quality” modules. This was followed by successful advancement of the“GCTP (Good Gene, Cellular, and Tissue-based Products Manufacturing Practice)” module in June. In August, the final module, “Reliability”, was completed smoothly. Oncolys BioPharma remains committed to submitting the marketing authorization application for OBP-301 by the end of December.
▲ Announced on May 13, May 23, June 24, and August 5, 2025
6. Oncolys BioPharma submitted an application for manufacturing and marketing approval (i.e., a New Drug Application, NDA) to the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan on December 15, 2025.
▲ Announced on December 15, 2025
*The “Sakigake Comprehensive Assessment Consultation” is a prior review mechanism under the Sakigake Designation System. It is conducted before the submission of a new drug approval application, aiming to evaluate data related to five modules: “Clinical”, “Non-clinical”, “Quality”, “Reliability” and “GCTP (Good Gene, Cellular, and Tissue-based Products Manufacturing Practice)”. This process helps identify and resolve potential issues in the review in advance, thereby shortening the overall review timeline.