
OBP-301 (Telomelysin) is a genetically modified human adenovirus type 5 with oncolytic potency, which is able to selectively replicate in cancer cells and to destroy cancer cells.
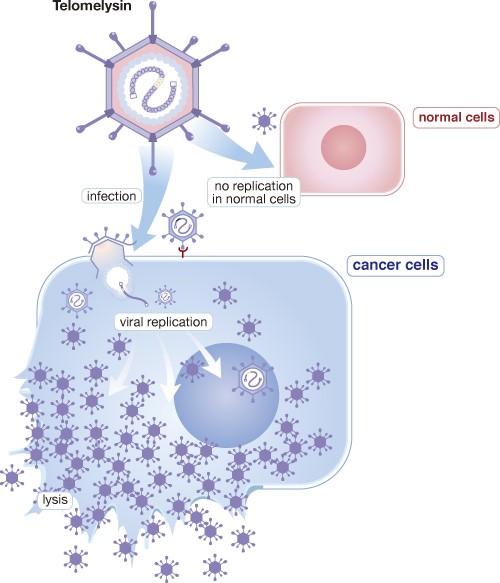
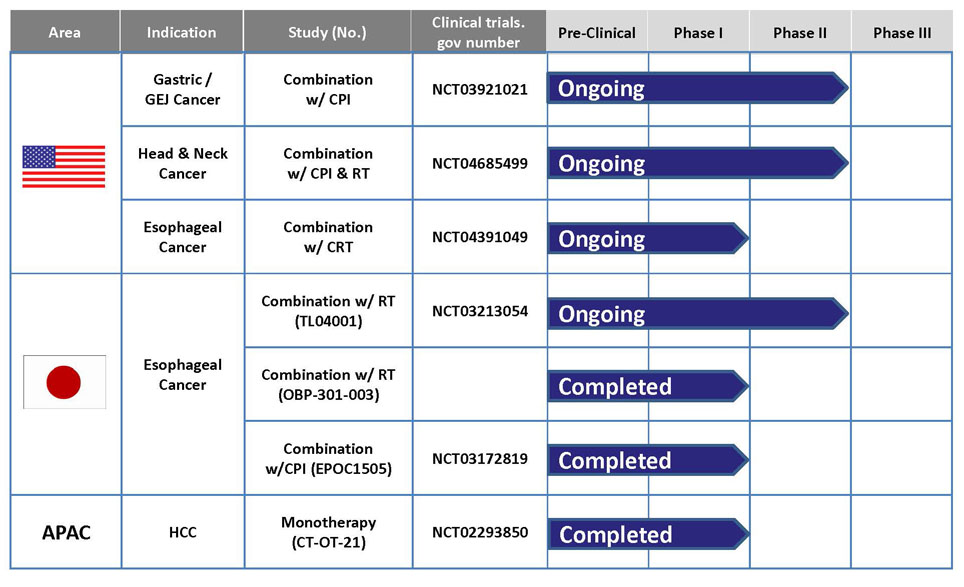
OBP-301 is co-developed by MBC and Oncolys BioPharma Inc. in Japan. At beginning, Oncolys tested its safety under Phase I dose-escalation design in the United States targeting patients with various solid tumors in 2008. A phase I study in patients with hepatocellular carcinoma has been conducted in Taiwan and Republic of Korea by MBC It was completed in 2020. Besides, Oncolys has completed a Phase I study in patients with esophageal cancer who received radiotherapy concurrently in Japan. Oncolys is conducting Phase II esophageal cancer clinical trial in Japan. And aim to file for Approval in Japan in 2024. Other ongoing clinical studies of OBP-301 include a Phase II study in combination with immune checkpoint inhibitors (ICI) for patient with gastric cancer/gastroesophageal junction cancer and a phase I study in combination with chemoradiotherapy for esophageal cancer patients in the United States.
OBP-301 (Telomelysin) & Current Development Status
For more information please refer to Oncolys website:https://www.oncolys.com/en/