
Globally, the incidence, mortality and burden of cancer continue to rise. As compared to the 2012 data, the number of incident cases and deaths in 2018 increased 4 million and 1.5 million. Eighteen million incident cases and 9.6 million deaths of cancer were reported by the cancer statistics 2018. Furthermore, it has been estimated that the incident cases of cancer will be increased to 29.3 million dramatically in 20 years, making cancer a global killer.
According to GLOBOCAN 2018, the most common new cases in both sexes were lung cancer, followed by breast cancer, colorectal and prostate cancer, with an incidence of 11.6%, 11.6% 10.2% and 7.1% of all new cancer cases, respectively. Lung cancer (18.4%) remained the top 1 cause of cancer deaths, followed by colorectal cancer (9.2%), stomach cancer (8.2%) and liver cancer (8.2%).
In Taiwan, liver cancer is the 2nd leading cause of cancer deaths with around 8000 deaths per year reported by MOHW. The most common type of liver cancer is hepatocellular cancer (HCC), which is mainly caused by chronic viral infection in Taiwan. Worldwide, the incidence of HCC is also rising rapidly for the past 2 decades. Up to date, the curative therapy for HCC are surgery and radiofrequency ablation (RFA) therapy. However, recurrence and deaths due to disease progression are frequent. Therefore, adaptive therapy for preventing post-operative recurrence for HCC is an unmet medical need.
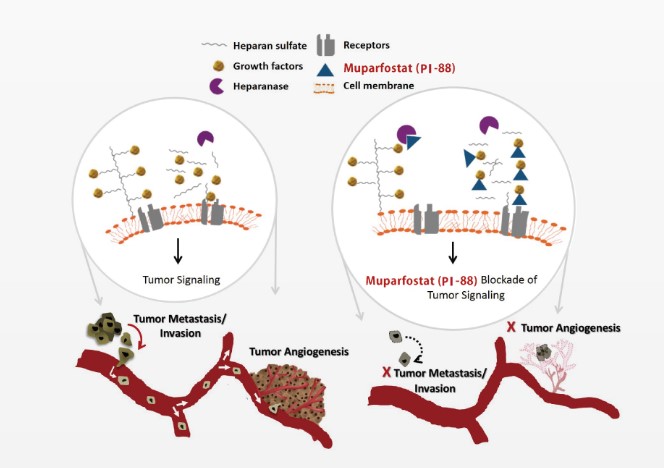
PI-88 consists of a mixture of highly sulfated monophosphorylated mannose oligosaccharides from the chemical sulfation of the hydrolysed phosphomannan produced by the yeast Pichia holstii, which has been demonstrated to inhibit angiogenesis and tumor metastasis in animal models. Several clinical studies of PI-88 had been successfully conducted by MBC in compliance with U.S. FDA guidelines, and application global guidance. These studies included Phase I and Phase II dose finding/feasibility studies to evaluate the safety profiles and preliminary efficacy of PI-88 in patients with various tumor types (e.g., multiple myeloma, hepatocellular carcinoma, lung cancer, and prostate cancer). The development of PI-88 has moved into Phase III following the results observed in early stage clinical studies. MBC has completed a large-scale, global Phase III study of PI-88 in patients with HCC in Asia including Taiwan, Korea, China and Hong Kong. MBC owns the full patent and global commercialization rights of PI-88, including the development, manufacture, and sublicense.
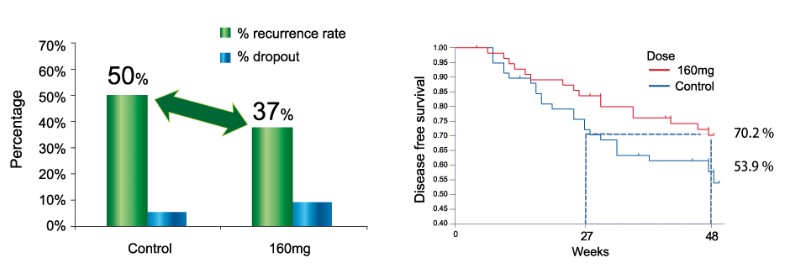
A Phase II clinical study (CT-PI-21) was conducted to investigate the safety and preliminary efficacy of PI-88 as adjuvant therapy in patients receiving a curative resection for HCC and the results had been published on Journal of Hepatology (vol: 50, 958-968, 2009). In this study, the recurrent rate was only 37% in patients receiving PI-88 at a dose of 160 mg/day, as compared with 50% in untreated control group. Besides, the time to recurrence was improved by 78% in the 160 mg/day group compared with the untreated control group (47 weeks vs. 24 weeks) at the 36th percentile. These results supported the use of PI-88 as an adjuvant therapy in patients with HCC following curative resection.
In order to determine the long-term prognosis in these patients, a follow-up observational study was launched, and the results showed that PI-88 at 160 mg/day delayed the onset of HCC recurrence, and provided clinically significant survival advantage especially in patients with multiple tumors or a single tumor ≥ 2 cm and hepatitis B or C infection for up to 3 years after treatment (56.8% improvement in disease-free survival, P <0.05). The study results had been published on World Journal of Gastroenterology (vol: 20, 11384-11393, 2014).
A Phase III clinical study (CT-PI-31) entitled “A Prospective, Randomized, Double-blind, Placebo controlled, Parallel-group, International Multicenter Phase III Trial Of PI-88 In the Adjuvant Treatment Of Subjects With Hepatitis Virus Related Hepatocellular Carcinoma After Surgical Resection (PATRON)” was initialized in 2011. The aim of this study was to investigate the efficacy and safety of PI-88 as adjuvant therapy to prevent recurrence in patients with HCC after curative resection. The recruitment of PATRON was closed in 2014 with 520 patients randomly assigned to receive 160 mg of PI-88 or placebo from 25 medical centers in Taiwan, Korea, China and Hong Kong. The results indicated that PI-88 was well-tolerated with acceptable safety profiles, and the disease-free survival was superior to placebo group in patients with microvascular invasion (around 41% of the enrolled patients). An extended study in a retrospective setting (CT-PI-31F) in patients participated in PATRON study was completed at the end of year 2019.
In December 2019, PI-88 was licensed to China Cellxpert Biotechnology Co., Ltd. for the research, development, manufacture, marking and reauthorization of globally right (but excluding Taiwan). PI-88 will be conducted by Cellxpert Biotechnology Co., Ltd. in the future.